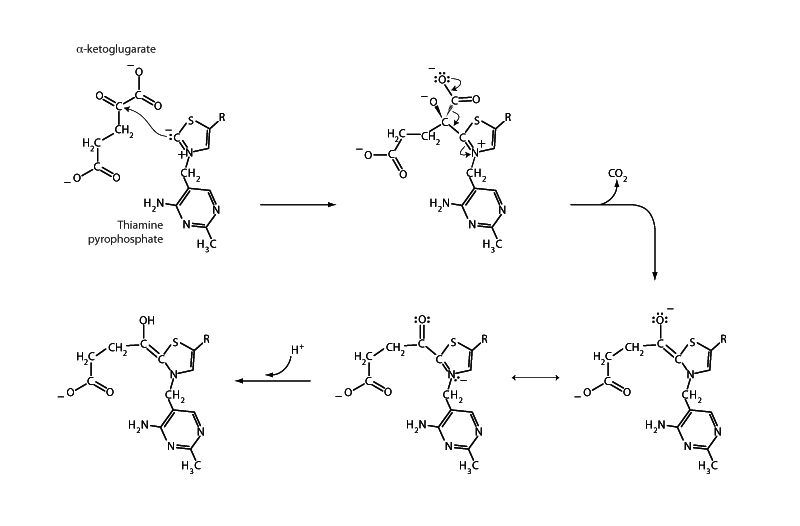
The multienzyme complex α-ketoglutarate dehydrogenase of the citric acid cycle catalyzes the conversion of α-ketoglutarate into succinyl CoA through oxidative decarboxylation. The α-ketoglutarate dehydrogenase complex possesses the same subunit structure and uses the same coenzymes as the pyruvate dehydrogenase complex. These coenzymes are thiamine pyrophosphate, lipoamide, coenzyme A, FADH2, and NAD+. The figure above depicts the decarboxylation step of this enzymatic mechanism. (Complete mechanism of the pyruvate dehydrogenase complex)
At this stage in our discussion we are focusing on the decarboxylation step of α-ketoglutarate dehydrogenase in order to appreciate the role of thiamine pyrophosphate in this mechanism. Remember that β-keto acids undergo decarboxylation quite easily, because the immediate product of decarboxylation is a resonant stabilized enolate anion. TPP creates the same type of scenario upon binding with an α-keto acid like pyruvate or α-ketoglutarate because the product of decarboxylation is a resonant stabilized imine-enamine anion.